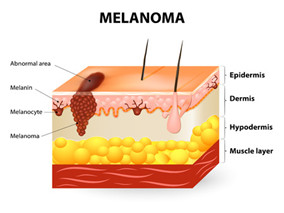
NICE拒绝将Vemurafenib用于黑色素瘤患者
6月19日消息 - 环球医学据悉,英国国立健康和临床优化研究所(Nice)决定不推荐Vemurafenib作为黑色素瘤患者的治疗选择,理由是该药价格太过昂贵,这一决定已受到皮肤癌患者支持小组的批评。
Factor 50公司敦促Nice重新考虑其不推荐该药物的决定,因为此药可能是一个潜在的救命药,该公司补充说,该决定就像下达判处死刑的命令。
Vemurafenib由罗氏公司生产制造,其花费约为每患者每周1750美元,虽然罗氏公司愿意以未公开的折扣价为NHS提供该药,但Nice认为该药不具成本效益。
“自20世纪70年代起就应用的标准治疗是无效的,拒绝用该药治疗患者,无异于给这些患者判了死刑。Nice已拒绝批准另一种将可大大改变黑色素瘤患者生活的药物,对此我感到惊讶,并深感忧虑,”Factor 50公司的首席执行官Gill Nuttall说。(环球医学)
原文:
NICE Rejects Vemurafenib for Melanoma Citing Costs
The National Institute of Clinical Excellence (Nice) decision not to recommend Vemurafenib as a treatment option for melanoma patients since it is too expensive has attracted criticism from a skin cancer patient support group.
Factor 50 has urged Nice to reconsider its decision to not recommend the drug as it could prove to be a potentially lifesaving drug and added that the decision was same as passing a death sentence.
Vemurafenib, manufactured by Roche, costs around 1,750 dollars per patient per week and though Roche was willing to offer an undisclosed discount for the NHS, Nice decided that it was not cost effective.
“Standard treatments that have been available since the 1970s are ineffective and to deny this drug to patients, is tantamount to passing them a death sentence. I am astonished and deeply worried that Nice has not given approval to yet another drug which will significantly alter the lives of melanoma patients,” Factor 50’s chief executive officer Gill Nuttall said.
相关链接:http://www.medindia.net/news/nice-rejects-vemurafenib-for-melanoma-citing-costs-102735-1.htm
- 评价此内容
- 我要打分
近期推荐
热门关键词
合作伙伴
Copyright g-medon.com All Rights Reserved 环球医学资讯 未经授权请勿转载!
网络实名:环球医学:京ICP备08004413号-2
关于我们|
我们的服务|版权及责任声明|联系我们
互联网药品信息服务资格证书(京)-经营性-2017-0027
互联网医疗保健信息服务复核同意书 京卫计网审[2015]第0344号

 会员登录
会员登录

